
| Home |
| Effectiveness and Tolerability of Every-Other-Day Rosuvastatin Dosing in Patients with Prior Statin Intolerance James M Backes, PharmD; Carmelo V Venero, MD; Cheryl A Gibson, PhD; Janelle F Ruisinger, PharmD; Patricia A Howard, PharmD FCCP BCPS; Paul D Thompson, MD; Patrick M Moriarty, MD Ann Pharmacother. 2008;42(3):341-346. ©2008 Harvey Whitney Books Company |
Background: Statins are generally well tolerated, but some patients discontinue
therapy secondary to adverse effects. Dosing a statin (rosuvastatin)
every other day (EOD) may provide significant lipoprotein changes while
avoiding common adverse effects in this statin-intolerant population.
Objective: To determine the effect and tolerance of EOD rosuvastatin in patients previously intolerant to statin therapy.
Methods: We performed a retrospective analysis of patients treated with EOD
rosuvastatin at 2 lipid specialty clinics: the University of Kansas
Lipid, Atherosclerosis, and LDL-Apheresis Center and the Hartford
Hospital Cholesterol Management Center. Approximately 2600 charts were
reviewed to identify patients receiving rosuvastatin EOD who previously
had experienced statin intolerance. Fifty-one patients were eligible
for the analysis, which evaluated changes in the lipid profile, the
number achieving their low-density lipoprotein cholesterol (LDL-C)
goals, and the percent tolerating rosuvastatin EOD. Laboratory data
were assessed immediately prior to rosuvastatin EOD therapy and at the
first follow-up.
Results: Myalgias (76.5%) and increased
transaminase levels (19.5%) were the most common causes of prior statin
intolerance, but 72.5% (37/51) of patients were able to tolerate the
EOD therapy (mean dose 5.6 mg) regimen for 4 ? 2.9 (mean ? SD) months.
Mean LDL-C decreased 34.5% (p < 0.001) in the patients who tolerated
the regimen, enabling approximately 50% to achieve their LDL-C goal.
All patients who were considered to be intolerant to rosuvastatin EOD
therapy (27.5%; 14/51) reexperienced the symptoms of their prior statin
intolerance.
Conclusions: Treating patients intolerant to
statins with rosuvastatin EOD was tolerated by the majority of patients
and reduced LDL-C in our study. This dosing strategy may be useful in
patients intolerant to once-daily statin dosing, although such an
approach has not been documented to reduce cardiovascular events.
Hydroxymethylglutaryl coenzyme A reductase inhibitors (statins) are well established as treatment for lowering low-density lipoprotein cholesterol (LDL-C) and reducing cardiovascular events. Overall, these agents have a remarkable safety profile, although some patients are intolerant of them.
Common reasons for discontinuation of statin therapy include elevated hepatic enzyme levels, gastrointestinal complaints, and "statin myopathy," which includes myalgia, weakness, cramping, and rhabdomyolysis.[1] Nonspecific muscle complaints or joint pain without elevated creatine kinase levels have been reported in approximately 5% of clinical trial participants;[2] however, the range is wide (0.3-33%).[3] An observational study, the PRIMO (Prediction of Muscular Risk in Observational conditions), reported muscle complaints in 10.5% of the 7924 statin-treated subjects,[4] suggesting that statin-induced myopathy is considerably more frequent in clinical practice.
A number of other drugs (eg, bile acid resins, ezetimibe, niacin) are available to reduce LDL-C in patients who are statin intolerant; however, these agents are less effective compared with statin therapy.[5] Moreover, the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP-III) Update emphasized LDL-C reductions of at least 30-40% and the option of more aggressive LDL-C goals for patients considered at high-risk for a cardiovascular event.[6] These factors produce the common clinical challenge of achieving an aggressive LDL-C goal in a high-risk patient intolerant to statin therapy. Thus, additional options are essential for patients unable to tolerate typical statin dosing in order to achieve the desired LDL-C goal.
The myalgias and elevation in hepatic enzymes caused by statin therapy are considered to be dose-dependent adverse effects.[7-10] Additionally, moderate-to-high statin doses possess a rather flat dose response relative to low doses. The lowest approved daily doses of statins will typically reduce LDL-C by 20-40% compared with moderate and high doses, which achieve only an additional 6% LDL-C reduction with each doubling of dose.[5] We theorized that by using low doses of a statin (rosuvastatin) every other day (EOD), a significant LDL-C reduction may still be achieved while also avoiding the dose-dependent adverse effects. Rosuvastatin appears to be a rational choice for markedly improving lipoprotein levels in statin-intolerant patients because of its high potency, long half-life, and lack of CYP3A4 metabolism.
Studies evaluating the effect and tolerance of alternative statin dosing are limited. A number of trials have demonstrated efficacy with alternate-day statin dosing,[11-15] including rosuvastatin;[15] however, the study populations included patients without previous intolerance to statins. A few anecdotal case reports have described efficacy and improved tolerance of rosuvastatin when dosed once weekly and thrice weekly. We previously reported on a subgroup of patients who demonstrated significant LDL-C reductions and better tolerance with EOD statin dosing, but the sample was small and included both atorvastatin and rosuvastatin.[16] The study reported here examined the effect and tolerance of EOD rosuvastatin therapy in a considerably larger cohort.
Approximately 2600 clinic charts were reviewed at 2 lipid-specialty clinics, the University of Kansas Lipid, Atherosclerosis, and LDL-Apheresis Center and the Hartford Hospital Cholesterol Management Center, to identify patients who received rosuvastatin EOD after previously experiencing intolerance to daily statin treatment. This retrospective protocol was approved by each hospital's institutional review board prior to record review.
Patients were included if they had previous documentation of statin intolerance, received rosuvastatin EOD for at least one month, and had reports of lipid levels immediately before and after EOD treatment. No sex or age limitations were included in our study. Subjects receiving other lipid-altering agents were included provided no other changes were made to their cholesterol regimen that could significantly impact the lipid profile. In contrast, no exclusions were made for individuals who may have altered their diet and exercise regimen during the time of the EOD therapy. Lastly, patients who reported nonadherence to any lipid-altering agent during the observation period were excluded from the study.
The primary outcomes for the study were the percent of patients who tolerated the alternate-day dosing regimen, changes in serum lipid levels from baseline to first follow-up, and the percent of patients who achieved their NCEP ATP-III and ATP-III Update LDL-C goals.
Tolerance of the EOD dosing was determined by patient feedback from chart reviews. Subjects who discontinued rosuvastatin secondary to an adverse effect were classified as EOD intolerant, and those who were able to continue therapy without drug-related complaints or who stated that rosuvastatin was tolerable were classified as EOD tolerant. Follow-up laboratory studies were performed at random times throughout the 48-hour dosing cycle.
Descriptive statistics (eg, mean ? SD) were calculated for all dependent variables. Continuous data are presented as mean ? SD. The t-test for paired samples was used to analyze plasma/serum lipid levels (changes in LDL-C, high-density lipoprotein cholesterol [HDL-C], and triglycerides) immediately before the EOD regimen and at the first follow-up. Values of p less than or equal to 0.05 (2-sided) were considered significant. All analyses were performed using SPSS for Windows, Release 14 (SPSS Inc., Chicago, IL).
A total of 51 patients met study criteria and were evaluated ( Table 1 ). The patients' past cardiovascular history included coronary heart disease (CHD), diabetes mellitus, and/or significant cardiac risk factors (hypertension, family history of CHD, metabolic syndrome). The most commonly reported previous intolerances to statins were myalgias or elevated hepatic transaminases. Most patients had shown previous intolerance to 1 to 3 different statins dosed once daily, whereas 6 participants had failed treatment with at least 4 statins.
The majority (72.5%; 37/51) of study patients were considered to be tolerant to EOD dosing with rosuvastatin. These patients experienced significant reductions in total cholesterol, triglycerides, and LDL-C from baseline to follow-up (all p < 0.001), whereas HDL-C did not change significantly ( Table 2 and Figure 1). The primary effect of EOD rosuvastatin therapy was a mean LDL-C reduction of 34.5%, which enabled 24 of 37 (64.9%) patients to achieve their NCEP ATP-III LDL-C goals. When including the more stringent, optional goals from the ATP-III Update (ie, <70 and <100 mg/dL), 17 of 37 (45.9%) met goal ( Table 3 ).[5,6] The mean dose of rosuvastatin was 5.6 ? 2.9 mg EOD (range 2.5-10).
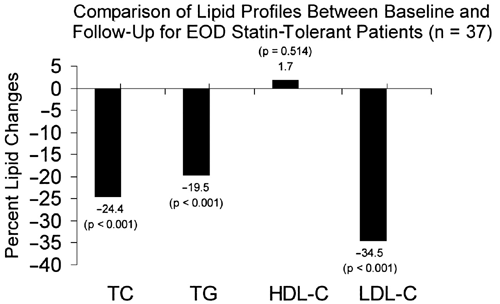
Figure 1.Percent change in major lipoproteins among subjects determined to be tolerant of the EOD rosuvastatin dosing regimen. Mean follow-up = 4.1 ? 2.9 months. EOD = every-other-day; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TC = total cholesterol; TG = triglycerides.
Fourteen (27.5%) of the 51 patients in the study were unable to tolerate rosuvastatin EOD. Of these patients, 10 had a return of their myalgias, 1 redeveloped gastrointestinal complaints, and 3 reexperienced their prior adverse effect (ie, fatigue, memory impairment, rash). A total of 10 (19.6%) patients in the entire study group with a prior history of statin-induced elevated hepatic enzymes were evaluated for EOD dosing. Upon their return to the clinic, 8 of these patients had hepatic enzyme levels within normal limits and 2 experienced a mild elevation (<80 U/L) in alanine aminotransferase but remained on therapy. Four patients with a prior history of myalgias had mildly to moderately elevated creatine kinase levels (250-750 U/L) at baseline; at follow-up, creatine kinase levels during EOD therapy were either similar or lower compared with baseline values.
These results suggest that many patients with a previous intolerance to statins can tolerate rosuvastatin EOD, attain significant LDL-C reductions, and achieve their LDL-C goal. Our findings are similar to previous data from case reports and small trials. We reported efficacy and improved tolerance with EOD statin dosing (rosuvastatin and atorvastatin) in a subset of patients who previously experienced myalgias with once-daily statin dosing.[16] Of the 15 patients who previously reported myalgias, 12 (80%) had a resolution of symptoms with EOD dosing. The 80% rate of tolerance in that investigation is similar to what we observed (72.5%; 37/51) in the present study. A recent case report involving 2 patients previously intolerant to atorvastatin indicated tolerance to rosuvastatin when dosed on Monday, Wednesday, and Friday, and LDL-C reductions of 20% and 38% after 6 weeks with 2.5-mg and 5-mg doses, respectively.[17] Lastly, we reported mean LDL-C reductions of 29% and tolerance in 80% of subjects (n = 10) previously intolerant to statin therapy when given rosuvastatin (mean dose 10 mg) once weekly.[18]
The average LDL-C reduction of 34.5% observed among the rosuvastatin EOD-tolerant group (mean dose 5.6 mg) in our study is consistent with the reduction shown in previous trials. Recently, a significant 39% reduction in LDL-C was achieved with rosuvastatin (10 mg) EOD among patients without a previous statin intolerance.[15] Further, we demonstrated similar LDL-C reductions (29%) in a subgroup of statin-intolerant patients who received either 5 or 10 mg of rosuvastatin EOD.[16] The doses of rosuvastatin used in our study were 2.5, 5, or 10 mg EOD (mean 5.6 mg). All doses used demonstrated significant LDL-C reductions; however, due to the small numbers in each subgroup, a comparative analysis was not performed.
We cannot specify why the EOD regimen was better tolerated by these patients. Possible explanations include the positive psychological effect of EOD treatment or lower plasma and muscle concentrations. Simply changing statins may also have contributed; Hansen et al.[19] reported that 43% of patients with previous statin-associated myopathy tolerated a different statin without a recurrence of symptoms. However, 4 patients in our study who had previously received rosuvastatin therapy daily and reported an adverse effect were able to tolerate the EOD regimen, thus making this explanation unlikely for all of the patients.
We elected to use rosuvastatin in an EOD regimen because its half-life of approximately 19 hours is the longest of available statins,[20] and it is the most potent statin at reducing LDL-C levels.[21] Atorvastatin also has a long half-life (~14 h) and has demonstrated effectiveness with EOD dosing.[16] Also, lovastatin, simvastatin, and atorvastatin are largely metabolized by CYP3A4, which is responsible for the conversion of many lipid-soluble agents to their hydrophilic forms for clearance and is a primary isoenzyme involved in many common drug interactions.[22] Rosuvastatin, in contrast, is primarily cleared renally and is minimally metabolized (<10%) by the less common CYP2C9 isoenzyme, and thus is potentially less likely to be implicated in common drug interactions.[22]
There are several limitations to this study. Our data were not prospectively obtained and the design was observational, without placebo controls. The sample size is small and myalgia was not objectively assessed in these patients, but rather was determined by self-report. Follow-up lipid levels were not determined at standard time points and consequently could both underestimate or overestimate the 48-hour lipid levels in these patients. Also, patients were not excluded if changes were made in nonpharmacologic therapies such as diet and exercise. Finally, no outcome studies have used such an alternate-day design; therefore, large, randomized, controlled trials are needed to confirm our findings. Because of these limitations, we cannot claim that reductions in lipid levels with this regimen will reduce cardiac events. Nevertheless, it is our hypothesis that patients are best treated with statins EOD rather than not treated at all.
Rosuvastatin EOD therapy was tolerated in the majority of patients with previous statin intolerance. Among patients who were EOD tolerant, rosuvastatin provided significant reductions in total cholesterol, LDL-C, and triglycerides while achieving LDL-C goals in many of the subjects. Such a regimen may be an effective therapeutic option for individuals who have previously been intolerant to traditional daily statin dosing.
N 51 Sex, n (%) male 23 (45.1) female 28 (54.9) Age, y (mean ? SD) 58.5 ? 10.5 Race, n (%) black 7 (13.7) white 40 (78.4) other 4 (7.8) BMI, kg/m2 (mean ? SD)a 28.64 ? 4.39 Waist circumference, in. (mean ? SD)a 37.05 ? 5.57 Major risk factors, n (%) family historyb 25 (49) hypertension 30 (58.8) smoking (current/past) 3 (5.8)/13 (25.5) HDL-C <40 mg/dL 8 (15.7) metabolic syndrome 22 (43.1) CHD, n (%) 11 (21.6) Diabetes mellitus, n (%) 11 (21.6) CVA, n (%) 4 (7.8) ATP-III Update risk category, n (%)6 highc 20 (39.2) moderately highd 8 (15.7) moderatee 17 (33.3) lowf 6 (11.7) Type of previous statin intolerance, n (%)g myalgias 39 (76.5) elevated hepatic transaminases 10 (19.6) other 5 (9.8) Concomitant lipid-altering drugs, n (%) 47 (92.2) omega-3 fatty acids (fish oil) 34 (66.6) ezetimibe 33 (64.7) BAR 11 (21.5) niacin 5 (9.8) fibrate 3 (5.9) otherh 12 (23.5) Previous number of intolerable statins, n (%) 1 21 (41.2) 2 13 (25.5) 3 11 (21.5) 4 5 (9.8) 5 1 (2) Previous intolerances to individual statins, ni atorvastatin 43 cerivastatin 2 fluvastatin 2 lovastatin 9 pravastatin 17 rosuvastatin 10 simvastatin 22 ATP = Adult Treatment Panel; BAR = bile acid resin; BMI = body mass index; CHD = coronary heart disease; CVA = cerebral vascular accident; HDL-C = high-density lipoprotein cholesterol.
an = 44.
bIndicates a family history of premature CHD (CHD in male first-degree relative <55 y of age; CHD in female first-degree relative <65 y of age).
cIncludes CHD or CHD risk equivalent, 10-y risk >20%.
dIncludes ?2 risk factors, 10-y CHD risk 10-20%.
eIncludes ?2 risk factors, 10-y CHD risk <10%.
fIncludes 0-1 risk factors.
gThree patients had experienced intolerance more than once; therefore, intolerance totals are >100%.
hIncludes plant stanol/sterols, policosanol, flaxseed oil.
iDosed daily.
Lipoprotein Baseline Mean ? SD (range) Follow-Upb Mean ? SD (range) p Value TC (mg/dL) 250.08 ? 36.54
(190-343)189.43 ? 36.16
(113-264)<0.001 TG (mg/dL) 159.16 ? 73.83
(49-340)128.43 ? 70.57
(35-394)<0.001 LDL-C (mg/dL) 164.59 ? 31.17
(118-234)107.57 ? 28.94
(62-171)<0.001 HDL-C (mg/dL) 58.95 ? 13.74
(30-78)59.89 ? 16.38
(33-95)0.514 HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TC = total cholesterol; TG = triglycerides.
an = 37.
bAverage time to follow-up 4.1 ? 2.9 months.
Risk Category n (%) LDL-C Goal, n (%) <70 mg/dL <100 mg/dL <130 mg/dL <160 mg/dL Higha 17 (45.9) 3 (17.6)e,6 9 (52.9)f,5 Moderately highb 4 (10.8) 2 (50)e,6 3 (75)f,5 Moderatec 11 (29.7) 8 (72.7)f,5 Lowd 5 (13.5) 4 (80)f,5 ATP = Adult Treatment Panel; CHD = coronary heart disease; LDL-C = low-density lipoprotein cholesterol.
aIncludes CHD or CHD risk equivalent, 10-y risk >20%.
bIncludes ?2 risk factors, 10-y CHD risk 10-20%.
cIncludes ?2 risk factors, 10-y CHD risk <10%.
dIncludes 0-1 risk factors.
eIndicates optional goal from ATP-III Update.
fIndicates goal from ATP-III.
|
James M Backes, PharmD, Department of Pharmacy Practice, Lipid, Atherosclerosis, Metabolic and LDL-Apheresis Center, University of Kansas Medical Center, 3901 Rainbow Blvd., Kansas City, KS 66160, fax 913/588-2355, E-mail: jbackes@kumc.edu .