
| Home |
| News 24/03/2544 |
| Extended-Release Niacin Safe for Diabetics With Uncontrolled Hypercholesterolemia |
| Long-Term Use of ERT Increases Risk of Ovarian Cancer Death in US |
| Strategic Management of Pharmaceutical Expenses Rita Shane [Am J Health-Syst Pharm 58(05):406-409, 2001. ? 2001 ASHP, Inc.] |
| Interrelationships among Mortality Rates, Drug Costs, Total Cost of Care, and Length of Stay in United States Hospitals: Summary and Recommendations for Clinical Pharmacy Services and Staffing C. A. Bond, Pharm.D., FASHP, FCCP, Cynthia L. Raehl, Pharm.D., FASHP, FCCP, Department of Pharmacy Practice, School of Pharmacy, Texas Tech University Health Sciences Center-Amarillo, Amarillo, Texas; and Todd Franke, Ph.D., Department of Biostatistics and Biometrics, School of Public Policy and Social Research, University of California at Los Angeles, Los Angeles, California [Pharmacotherapy 21(2):129-141, 2001. © 2001 Pharmacotherapy Publications, Inc.] |
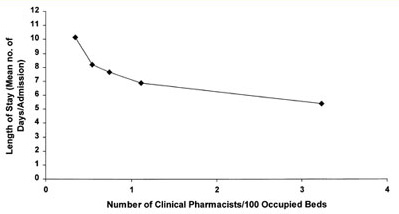
Figure 1. Clinical pharmacist staffing and length of hospital stay.
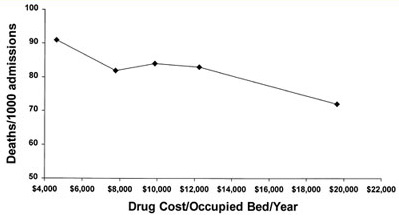
Figure 2. Hospital deaths/1000 admissions and drug costs.
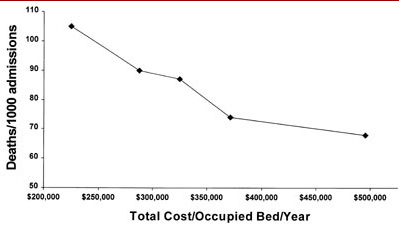
Figure 3. Hospital deaths/1000 admissions and total cost of care.
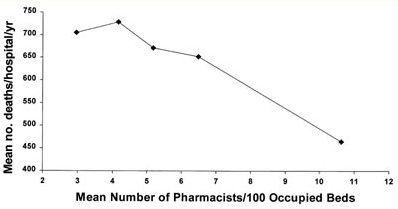
Figure 4. Pharmacist staffing and deaths/hospital.
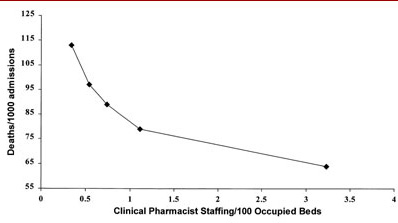
Figure 5. Clinical pharmacist staffing and hospital deaths/1000 admissions.The only pharmacy variable that was associated with positive outcomes with all four outcome measures was the number of clinical pharmacists/occupied bed. This tended to have the greatest association (slope) with reductions in mortality rate, drug costs, and length of stay. It also had the second highest association (slope) with reductions in total cost of care. No individual clinical pharmacy service was associated with all four outcome measures. Three services were associated with three outcome measures: pharmacist-provided drug information, drug protocol management, and admission drug histories. Pharmacist participation on medical rounds was associated with improvements with two outcome measures, total cost of care and length of stay. Five services were associated with improvements with one outcome measure: drug use review, in-service education, clinical research, ADR monitoring, and CPR team participation. Pharmacist-provided drug information, drug protocol management, and admission drug histories were associated with reductions in both drug costs and total cost of care. Pharmacist-provided clinical research and participation on the TPN team were associated with increased total cost of care.
aNot determined as part of the original analysis. See Table 4 for specific information on pharmacy staffing and mortality rates.
Mortality Rate[8]
(1029 hospitals)Drug Costs[9]
(934 hospitals)Total Cost of Care[10]
(1016 hospitals)Length of Stay
(1024 hospitals)Slope p Value Slope p Value Slope p Value Slope p Value Central clinical pharmacy services Drug-use evaluation -34871 0.001 In-service education -1148 0.016 Drug information -0.002 0.043 -1090 0.015 -11749402 0.003 Poison information Clinical research -0.008 0.0001 42922279 0.0001 Patient-specific clinical pharmacy services ADR monitoring -6599253 0.008 Pharmacokinetic consultations Drug therapy monitoring Drug protocol management -1065 0.049 -17423551 0.001 -1.30 0.008 TPN team participation 10789291 0.001 Drug counseling CPR team participation -0.002 0.039 Medical rounds participation -4770426 0.0001 -1.71 0.001 Admission drug histories -0.006 0.005 -1450 0.011 -6106570 0.017 Pharmacy staffing/occupied beds All pharmacists -0.0381 0.0185 Pharmacy administrators Xa Xa 46442 0.0001 -324890768 0.0001 Dispensing pharmacists Xa Xa 53299 0.0001 120000000 0.006 Clinical pharmacists Xa Xa -21809 0.018 -38864012 0.007 -26.59 0.001 Pharmacy technicians Xa Xa 54915 0.0001 R2 (actual) 22.4% 15.3% 48.9% 11.4%
aAll hospitals that offer the service.
Lower Deaths (actual)[8]
(1029 hospitals)Lower Drug Costs ($)[9]
(934 hospitals)Total Cost of Care ($)
(increase or reduction)[10]
(1016 hospitals)
Per Hospital All Hospitalsa Per Hospital All Hospitalsa Per Hospital All Hospitalsa Central clinical pharmacy services Drug use evaluation 1,119,810 1,005,589,542 In-service education 77,879 48,518,735 Drug information 3.89 10,463 430,580 90,852,346 5,226,128 1,212,461,747 Poison information Clinical research 11.63 21,125 (9,558,788)b (1,013,231,529)b Patient-specific clinical pharmacy services ADR monitoring 1,610,841 1,101,815,258 Pharmacokinetic consultations Drug therapy monitoring Drug protocol management 137,334 45,045,444 1,729,608 614,010,986 TPN team participation (3,211,355)b (1,027,633,638)b Drug counseling CPR team participation 2.1 5047 Medical rounds participation 7,979,721 1,212,917,508 Admission drug histories 8.61 3843 213,388 5,548,094 6,964,145 208,924,355
bIncrease in total costs associated with these services.
Services Associated with
Reduced Length of StayNo. (%) of
Hospitals Providing
the ServiceMean Reduction in
Length of Stay/Patient
in Hospitals
Offering the ServiceTotal No. of
Patient Days
Reduced/Hospital
Offering the ServiceTotal No. of
Patient Days Reduced
for All Hospitals
Offering the ServiceDrug protocol management 354 (34.6) 1.22 ± 0.91 432.67 ± 167.93 152,998.80 Medical rounds participation 153 (14.8) 1.34 ± 0.93 164.82 ± 88.51 25,178.46
R2
Types of Hospital Pharmacy Staff Slope Actual (%) Adjusted (%) Significance All pharmacists -0.101710 4.5 4.1 0.0001 Pharmacy administrators 0.190177 3.4 3.2 0.0001 Dispensing pharmacists -0.091700 3.6 3.3 0.0001 Clinical pharmacists -0.408114 10.1 9.8 0.0001 Pharmacy technicians -0.097564 3.2 2.8 0.0001