
| Home |
| News 15/07/2544 |
| Efficacy of Atorvastatin in Achieving National Cholesterol Education Program Low-Density Lipoprotein Targets in Women With Severe Dyslipidemia and Cardiovascular Disease or Risk Factors for Cardiovascular Disease: The Women's Atorvastatin Trial on Cholesterol (WATCH) Ruth McPherson, MD, PhD, FRCPC,a Carmella Angus, MSc,b Peter Murray, BS,b and Jacques Genest, Jr, MD, FRACP,c for the WATCH Investigators Ottawa, Ontario, Canada, and Montreal, Quebec, Canada. [Am Heart J 141(6):949-956, 2001. © 2001 Mosby-Year Book, Inc.] |
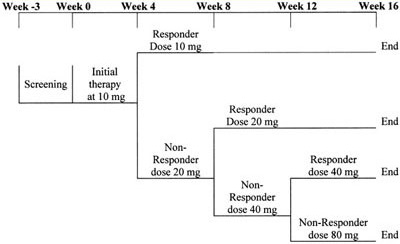
Figure 1. Atorvastatin treatment schedule: This was a 16-week study with a screening visit at week -3. All eligible patients with and without prior lipid treatment underwent a 3-week washout period on diet alone. At baseline (week 0) patients started treatment with atorvastatin 10 mg with a conditional titration to 20 mg at week 4, to 40 mg at week 8, and to 80 mg at week 12 dependent on the dose required to achieve NCEP LDL-C targets. Once the LDL-C target was reached, the patient remained at the target dose for the remainder of the study.
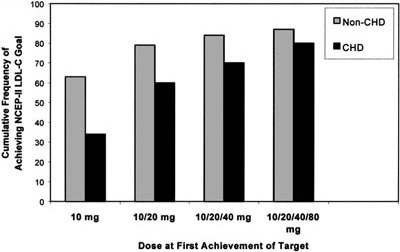
Figure 2. Cumulative frequency of achieving NCEP ATP II LDL-C target by atorvastatin dose and cardiac risk group. The majority of WATCH participants without CVD (63%) reached LDL-C targets with 10 mg of atorvastatin, 79% with up to 20 mg, and with maximal titration up to 80 mg atorvastatin 87% of women achieved LDL-C targets. For women with established CVD, 34% reached the LDL-C target of100 mg/dL (
2.6 mmol/L) with 10 mg and 60% with up to 20 mg of atorvastatin and with maximal titration up to 80 mg 80% of women with CVD achieved LDL-C target.
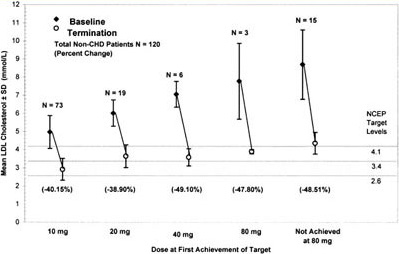
Figure 3. Mean LDL-C concentrations at baseline and termination by dose at first achievement of target for non-CVD patients. This figure shows both the mean baseline LDL-C concentrations as well as the mean LDL-C level of patients who achieved LDL-C targets at each titrated dose. Three parallel lines indicate LDL-C targets dependent on NCEP ATP II risk category. This figure shows a continuous increase in the mean baseline LDL-C at each titrated dose of atorvastatin with a parallel increase in the mean percent reduction of LDL-C. The mean percent reduction at 80 mg includes only 3 patients and therefore is not a reliable mean percent reduction at the 80 mg dose. Patients who did not achieve targets had a mean baseline LDL-C of 337 mg/dL (8.7 mmol/L).
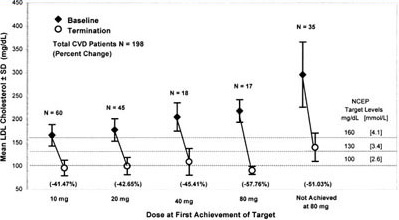
Figure 4. Mean LDL-C concentrations at baseline and termination by dose at first achievement of target for CVD patients. This figure shows both the mean baseline LDL-C concentrations as well as the mean LDL-C level of patients who had achieved LDL-C targets at each titrated dose. Three parallel lines indicate LDL-C targets dependent on NCEP ATP II risk category. The LDL-C target for the CVD group was 100 mg/dL (2.6 mmol/L). This figure shows a continuous increase in the mean baseline LDL-C at each titrated dose of atorvastatin with a parallel increase in the mean percent reduction of LDL-C at each dosage increase. Patients who did not achieve target had a mean baseline LDL-C of 298 mg/dL (7.7 mmol/L).
Non-CVD
(n = 120)CVD
(n = 198)Demographics Age (y) 54.7 ± 12.1 60.3 ± 8.7* BMI (kg/m2) 28.9 ± 5.3 28.1 ± 6.1 Abdominal circumference (cm) 90.9 ± 14.7 89.2 ± 13.5 Waist-to-hip ratio 0.85 ± 0.09 0.85 ± 0.07 SBP (mm Hg) 127 ± 18 131 ± 16 DBP (mm Hg) 79 ± 10 77 ± 8 White race (%) 95 95 Postmenopausal (%) 70 89* On HRT (%) 27 30 Risk factor prevalence (No. [%]) 55 y old/menopausal/no HRT
72 (60%) 149 (75%)* Family history or premature CVD 65 (54%) 111 (56%) Cigarette smoker 23 (19%) 58 (29%) Hypertension 51 (43%) 114 (58%) Diabetes mellitus 11 (9%) 21 (11%) HDL-C 35 mg/dL (0.9 mmol/L)
16 (13%) 24 (12%) HDL-C 60 mg/dL (1.6 mmol/L)
7 (6%) 30 (15%) <2 risk factors and no CVD 41 (34%) 2 risk factors and no CVD
79 (66%)
Data are expressed as mean ± SD. *P < .01 between groups.
Risk factor Mixed dyslipidemia
with obesityPresent
(n = 72)Absent
(n = 240)55 y old/menopausal/no HRT
56 (78%) 160 (67%) Family history of premature CVD 37 (51%) 135 (56%) Cigarette smoker 27 (38%) 53 (22%)* Hypertension 44 (61%) 119 (50%) Diabetes mellitus 14 (19%) 17 (7%)* HDL-C 35 mg/dL (0.9 mmol/L)
19 (26%) 21 (9%)* HDL-C 60 mg/dL (1.6 mmol/L)
2 (3%) 34 (14%)*
*P < .01 between groups.
Non-CVD (n = 120) CVD (n = 198) Baseline Final Baseline Final Cholesterol mg/dL 314.9 ± 67.1 210.3 ± 31.0* 299.3 ± 63.0 199.1 ± 34.6* mmol/L 8.1 ± 1.7 5.4 ± 0.8* 7.7 ± 1.6 5.0 ± 0.9* Triglycerides mg/dL 212.1 ± 87.6 169.8 ± 76.5* 226.0 ± 82.3 164.5 ± 63.7* mmol/L 2.4 ± 1.0 1.9 ± 0.9* 2.5 ± 0.9 1.9 ± 0.7* LDL-C mg/dL 226.2 ± 65.6 128.3 ± 31.2* 206.3 ± 60.9 111.1 ± 32.1* mmol/L 5.8 ± 1.7 3.3 ± 0.8* 5.3 ± 1.6 2.9 ± 0.8* HDL-C mg/dL 46.7 ± 9.9 48.8 ± 11.8 48.1 ± 11.8 48.4 ± 11.0 mmol/L 1.21 ± 0.26 1.26 ± 0.30 1.24 ± 0.30 1.25 ± 0.28 Apolipoprotein B (g/L) 2.10 ± 0.46 1.35 ± 0.24* 1.99 ± 0.43 1.24 ± 0.28*
Data are presented as mean ± SD. *P < .0001 versus baseline.
Mixed dyslipidemia and abdominal obesity Present (n = 72) Absent (n = 240) Baseline Final Baseline Final Cholesterol mg/dL 306.4 ± 55.6 200.6 ± 3.5* 305.3 ± 67.7 198.7 ± 34.9* mmol/L 7.9 ± 1.4 5.2 ± 0.9* 7.9 ± 1.8 5.1 ± 0.9* Triglycerides mg/dL 293.8 ± 55.5 216.9 ± 1.7* 199.3 ± 79.4 151.3 ± 59.6* mmol/L 3.3 ± 0.6 2.4 ± 0.8* 2.2 ± 0.9 1.7 ± 0.7* LDL-C mg/dL 206.9 ± 55.0 114.2 ± 1.6* 216.3 ± 65.6 119.1 ± 33.1* mmol/L 5.4 ± 1.4 3.0 ± 0.8* 5.6 ± 1.7 3.1 ± 0.9* HDL-C mg/dL 41.5 ± 8.5 44.1 ± 0.5* 49.3 ± 11.2 49.7 ± 11.1 mmol/L 1.07 ± 0.22 1.14 ± 0.27* 1.27 ± 0.29 1.28 ± 0.29 Apolipoprotein B (g/L) 2.10 ± 0.36 1.35 ±.23* 2.02 ± 0.47 1.27 ± 0.28*
Data are presented as mean ± SD. *P < .0001 versus baseline.
| Book Review How to Read a Paper: The Basics of Evidence Based Medicine, 2nd Edition |
| Melatonin Helps Sleep Quality in Diabetics, Facilitates Withdrawal From Benzodiazepines |
| US Drugstores Look Beyond Borders for Pharmacists |