
| Home |
| Treatment of Hepatitis B Vivek Raj, MD, MRCP (UK), Assistant Professor, Division of Gastroenterology and Hepatology, University of Arkansas for Medical Sciences and Central Arkansas Veterans Healthcare System, Little Rock, Arkansas [Clinical Cornerstone 3(6):24-36, 2001. ฉ 2001 Excerpta Medica, Inc.] |
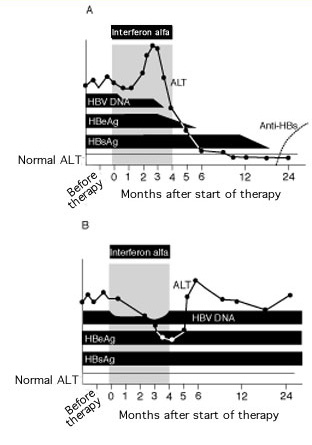
Figure 1. Serum alanine aminotransferase (ALT) and viral markers in patients with chronic hepatitis B virus (HBV) during and after 4-month course of interferon alfa. (A) Course in a patient with a sustained response. (B) Course in a patient with a poor response. HBeAg = hepatitis B e antigen; HBsAg = hepatitis B surface antigen; anti-HBs = antibody to HBsAg. Reprinted with permission from Hoofnagle JH, diBisceglie AM. The treatment of chronic viral hepatitis. N Engl J Med. 1997;336:347-356.
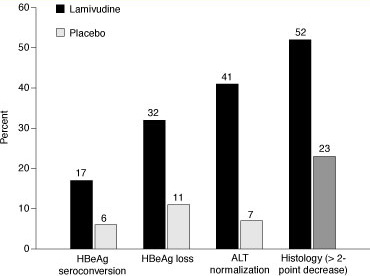
Figure 2. Response of hepatitis B virus (HBV) to treatment with lamivudine compared with placebo for 1 year. HBeAg = hepatitis B e antigen; ALT = alanine aminotransferase. Modified from Dienstag JL, Schiff ER, Wright TL, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256-1263.
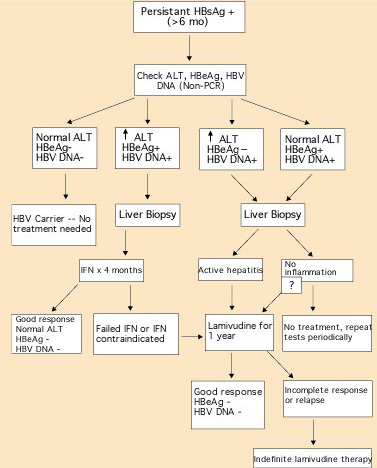
Figure 3. Algorithm for managing patients with hepatitis B virus (HBV). HBsAg = hepatitis B surface antigen; ALT = alanine aminotransferase; HBeAg = hepatitis B e antigen; PCR = polymerase chain reaction; IFN = interferon.
| Serologic Test | HBsAg | Anti-HBs | Anti-HBc | HBeAg | Anti-HBe | HBV DNA |
|---|---|---|---|---|---|---|
| Acute infection | ||||||
| Early phase |
|
|
|
|
|
|
| Window phase |
|
|
|
|
|
|
| Recovery |
|
|
|
|
|
|
| Chronic infection | ||||||
| Active replication |
|
|
|
|
|
|
| Low replication |
|
|
|
|
|
|
| Precore mutant |
|
|
|
|
|
|
| HBV vaccination |
|
|
|
|
|
|