
| Home |
| Beyond the Usual Strategies for Blood Pressure Reduction: Therapeutic Considerations and Combination Therapies |
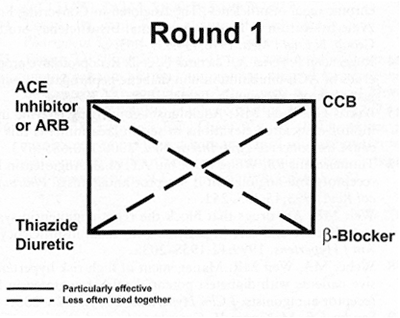
Figure 1. Initial or "Round 1" therapy for the hypertensive subject usually begins with one of the four major classes of drugs. An angiotensin receptor blocker (ARB) may be used in place of an angiotensin-converting enzyme (ACE) inhibitor for avoidance of ACE intolerance or ACE side effects. A calcium channel blocker (CCB) may also be appropriate in certain clinical situations. When a second drug is necessary, the most useful combinations are connected by solid lines.
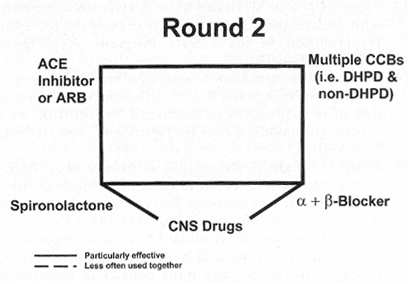
Figure 2. "Round 2" illustrates drug combinations that may be particularly useful in the difficult-to-control patient. It may occasionally prove necessary to combine a dihydropyridine (DHPD) calcium channel blocker (CCB) with a non-DHPD, such as diltiazem or verapamil. Angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker (ACE/ARB) combinations and alpha1/beta blocker combinations may be helpful, as may be the addition of a central alpha2 agonist, such as clonidine. Spironolactone may be especially efficacious for patients with increased plasma aldosterone levels (see text). CNS=central nervous system
|